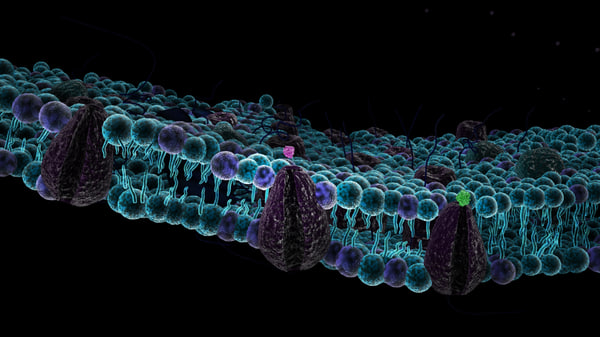
The cell membrane is the barrier that separates the cell from the external environment.
It is composed of 2 fat layers called a phospholipid bilayer

Polar Head (hydrophilic/water loving)& Nonpolar Tail(hydrophobic/water fearing)
The cell membrane is known to be selectively permeable (only selected molecules can pass through it or leave from it)

Attached within the cell (intracellular receptors) which are proteins receptors that allow specific molecules in and out of the cell.
When a substance (ligand) attaches to the correct receptor it is allowed in.
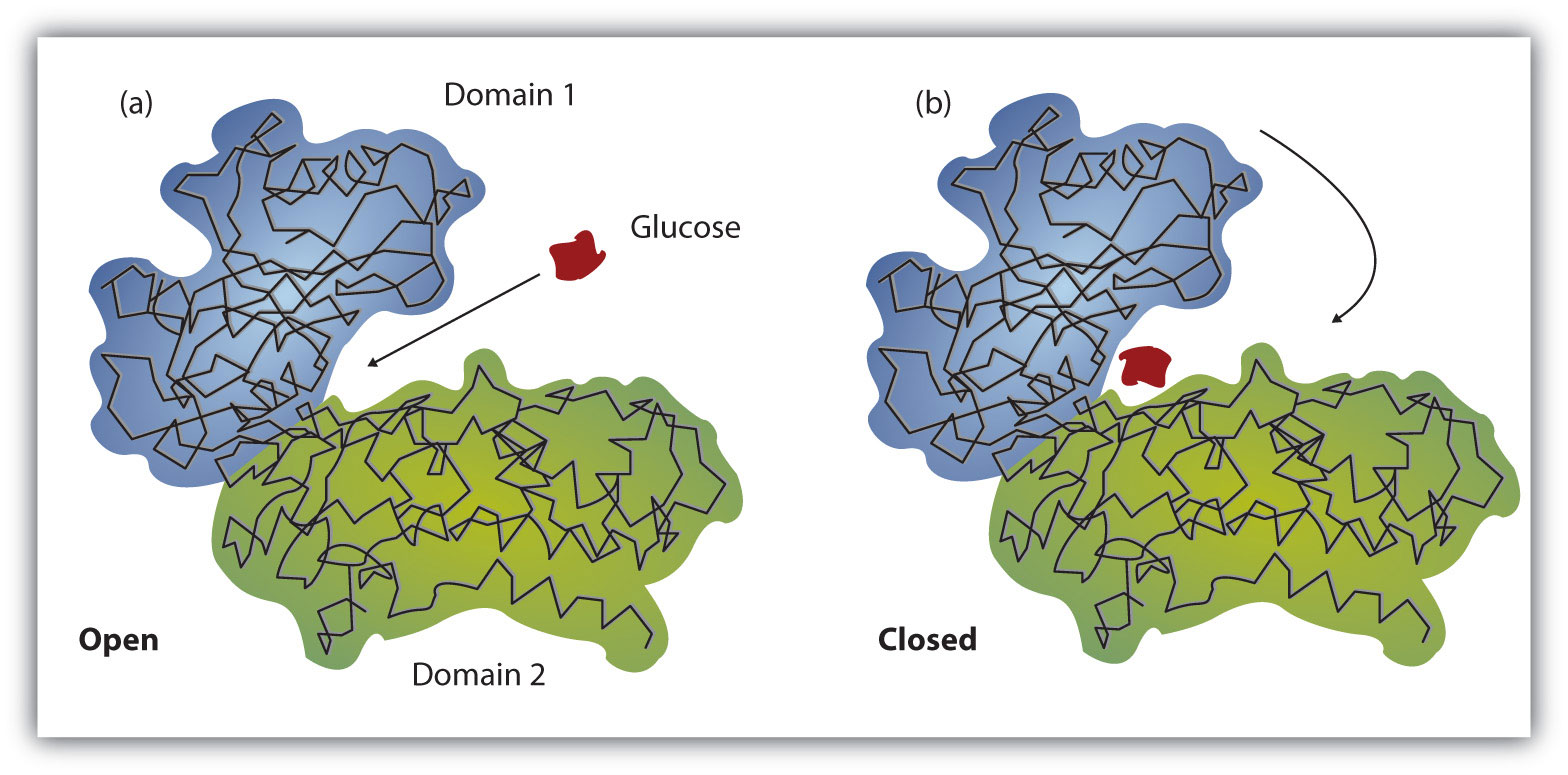
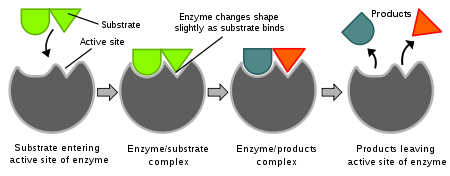
Membrane Receptors: are located in the membrane and binds to molecules that cannot cross the membrane, change in shape transmits the message to the cell interior
Take a look....
This is the Fluid Mosaic Model : describes the arrangements of molecules making up a cell membrane. (membrane is flexible like FLUID and has a variety of molecules like the variety of tyles in a mosaic.
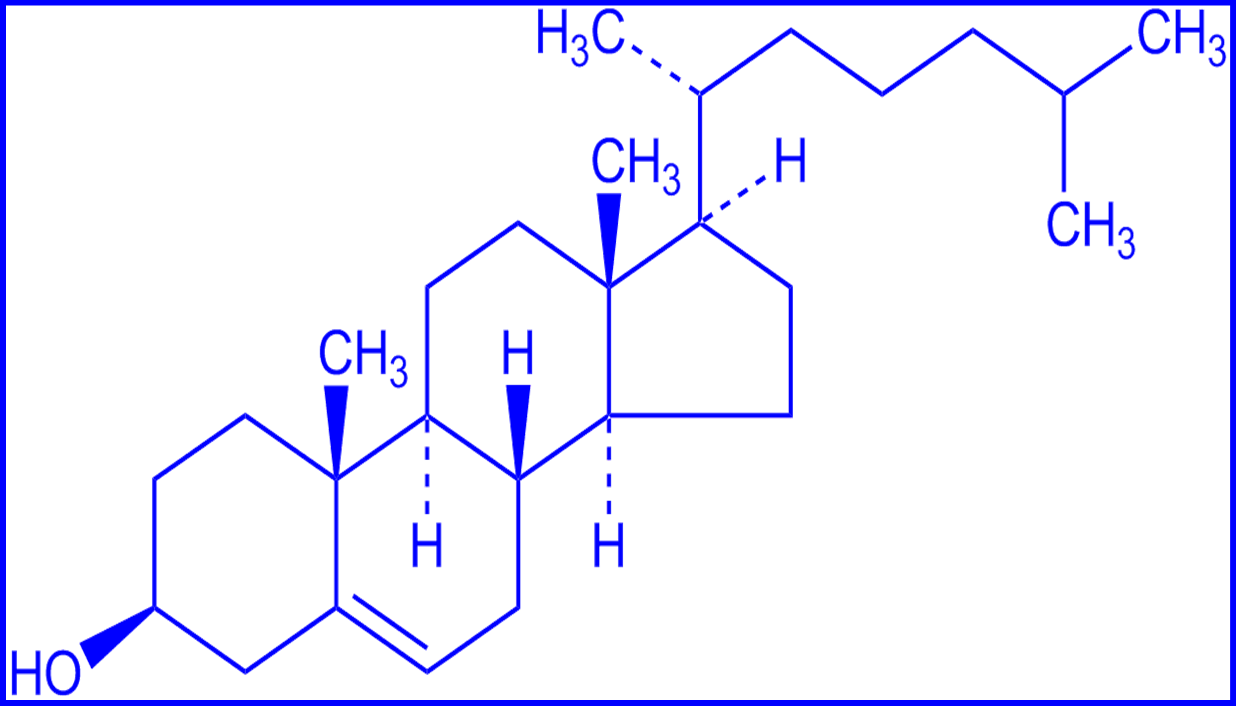
Other Molecules found in the membrane:
cholesterol: strengthens membranes
carbohydrates aid cell identification
Check it out!
Lets assess your knowledge....
Cell Membrane Quiz Section 3.3
Section 3.4
There are 2 methods of transporting molecules across a membrane....
Passive Transport
No ATP,Energy, required because molecules are moving from areas where there are many [High] to areas where there are less [Low] known as down a concentration gradient.
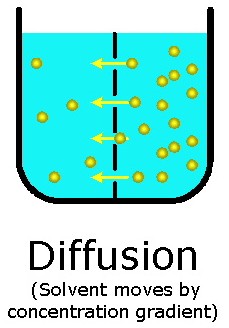
-Diffusion (molecules moving from [High] --> [Low])
-Osmosis (H2O moving from [High] --> [Low])

So why does this matter?...
(Review vocabulary solute vs solvent vs solution)
Your cells (animal cells) function at a specific concentration concentration. Too much/too little of a solution can have deadly effects


Lets assess your knowledge....
Section 3.5
Active Transport
ATP required because we are moving molecules against what they tend to normally do.
-Similar to facilitated diffusion but in the opposite direction [Low] --> [High] up a concentration gradient.


Two different ways of active transport are....
Endocytosis (into cell)

Exocytosis (out of cell)

Lets watch this video
Lets assess your knowledge....
Active Transport,Endocytosis,Exocytosis Sec 3.5