Changing substances to different substances by breaking or forming chemical bonds.
.
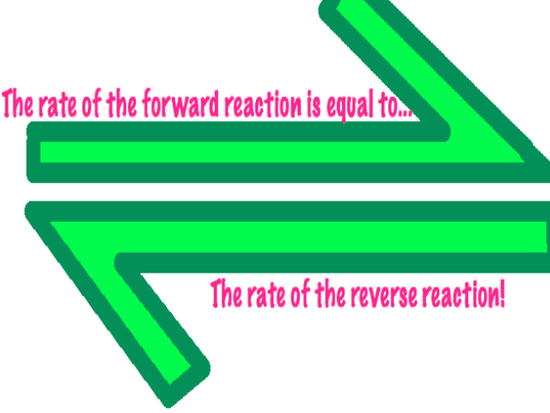
Reactants go in ----> Products come out
Example:
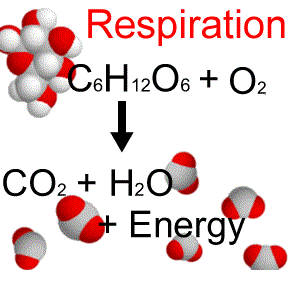

In order for reactions to occur energy is required known as activation energy.
Some reactions absorb energy than they release (Endothermic Reactions)

Some reactions release more energy than they absorb (Exothermic Reactions)

So why does this matter?
At this moment your body is going through many chemical reactions at a cellular level with the help of ENZYMES.
What are Enzymes?
All Enzymes are proteins but not all proteins are enzymes.
What????? It's kinda like....
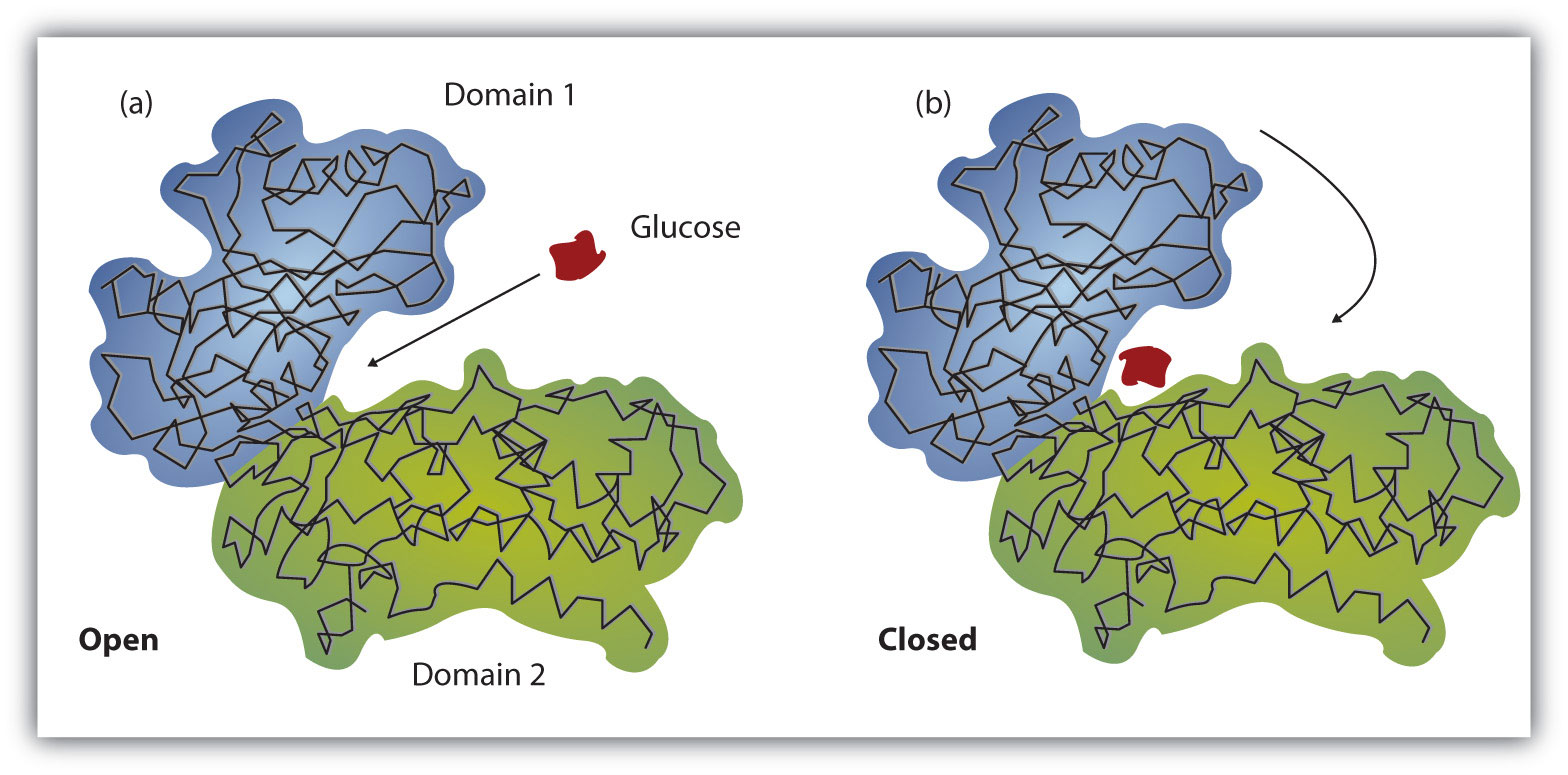
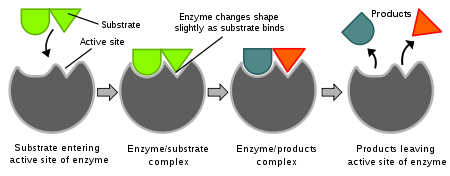
Enzymes are known as catalyst because they lower the energy needed for a reaction to take place.
Reactions happen at a faster pace with enzymes.
Check this out....

Some enzymes allow reactions to occur quick and require less energy but require a optimal temperature and/or pH.


Other enzymes break items down like Amylase in your saliva.
 Amylase is an enzyme that catalyzes starch into sugars in your mouth.
Amylase is an enzyme that catalyzes starch into sugars in your mouth.
Helpful tip: Enzymes can be identified by the ending of -ase. (ex: amylase, lipase, etc...) there are exceptions like pepsin and trypsin which are proteins used for digestion.
So how do enzymes work?....
Enzymes have a specific site where specific substances attach. So enzymes are specific kind of like a lock and key.


Lets assess your knowledge....Enzyme Quiz Section 2.4
For extra help please access the online book at my.hrw.com
(Access code needed)
For extra help please access the online book at my.hrw.com
(Access code needed)

No comments:
Post a Comment